23. Urine sodium
| Ward | Not stated | D.O.B/Age | 10/06/1958 |
| Consultant |
Urine sodium 224 mmol/L
Request form: hyponatraemia
Many patients present due to manifestations of other medical comorbidities, with hyponatremia being recognized only secondarily. Many medical illnesses, such as chronic heart failure, liver failure, renal failure, or pneumonia, may be associated with hyponatremia. Patients usually present with symptoms related to their primary illness.
Symptoms of hyponatremia range from nausea and malaise, with a mild reduction in the serum sodium, to lethargy, decreased level of consciousness, headache, and (if severe) seizures and coma. Overt neurologic symptoms most often are due to very low serum sodium levels (usually < 115 mmol/L), resulting in intracerebral osmotic fluid shifts and brain oedema.
Examination should include orthostatic vital signs and an accurate assessment of volume status. Volume status forms an integral part of assessment as it often guides assessment and treatment.
A full assessment for medical comorbidities is also essential, with particular attention to cardiopulmonary and neurologic components of the examination.

CT brain and CXR may be indicated if SIADH suspected.
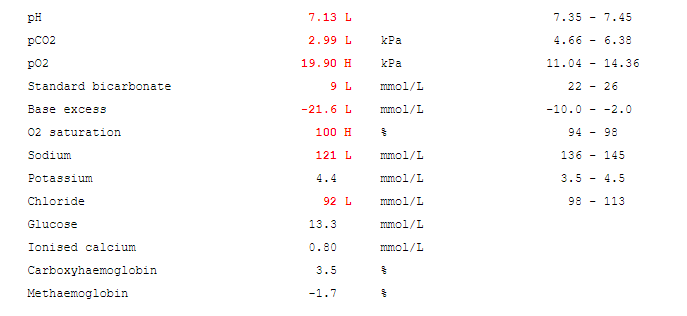
True hyponatraemia.
Hyponatremia can be classified according to volume status, as follows:
- Hypovolemic hyponatremia: decrease in total body water with greater decrease in total body sodium
- Euvolemic hyponatremia: normal body sodium with increase in total body water
- Hypervolemic hyponatremia: increase in total body sodium with greater increase in total body water
Hyponatremia can be further subclassified according to effective osmolality, as follows:
- Hypotonic hyponatremia
- Isotonic hyponatremia
- Hypertonic hyponatremia
There are three essential laboratory tests in the evaluation of patients with hyponatremia that, together with the history and the physical examination, help to establish the primary underlying etiologic mechanism: urine osmolality, serum osmolality, and urinary sodium concentration.
- Urine osmolality: essential to differentiate a deficiency in excreting free water vs primary polydipsia. Urine osmolality greater than 100 mOsm/kg indicates impaired ability of the kidneys to dilute the urine.
- Serum osmolality: differentiates between true hyponatremia and pseudohyponatremia. True hyponatraemia causes an decrease in serum osmolality.
- Urinary sodium: helps to differentiate between hyponatremia secondary to hypovolemia and the syndrome of inappropriate ADH secretion (SIADH). With SIADH (and salt-wasting syndrome), the urine sodium is greater than 20-40 mmol/L. With hypovolemia, the urine sodium typically measures less than 25 mmol/L.
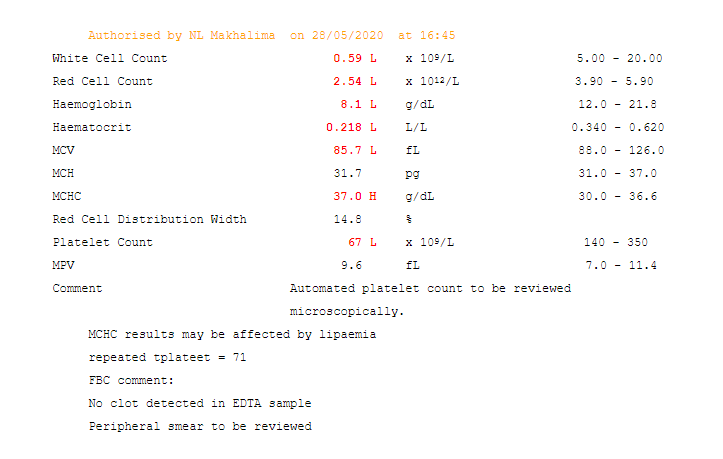
Ancillary testing may also help with differentiating SIADH from salt-wasting. Serum uric acid levels can be important supportive information (they are typically reduced in SIADH and also reduced in salt wasting). After correction of hyponatremia, the hypouricemia corrects in SIADH but remains with a salt-wasting process.